We developed and validated a deep learning model to determine the presence of hepatic steatosis using chest radiographs, which are widely performed during routine health checkups.
Paper
Performance of a Chest Radiograph-based Deep Learning Model for Detecting Hepatic Steatosis
Radiology: Cardiothoracic Imaging
https://doi.org/10.1148/ryct.240402
Author's Comments
Diagnosis of hepatic steatosis typically relies on ultrasound examinations; however, the availability of equipment and trained personnel can be limited. We focused on chest radiography, a more common and simple examination. While chest X-rays are often perceived as being primarily "for the lungs," we hypothesized that AI analysis of the information contained within these images could estimate the risk of hepatic steatosis. Our results suggest that the model may capture image features that are difficult for humans to distinguish. Although this model is intended as a screening aid, we hope it will lead to reduced patient burden and earlier detection, and we plan to continue our validation efforts.
Paper Overview
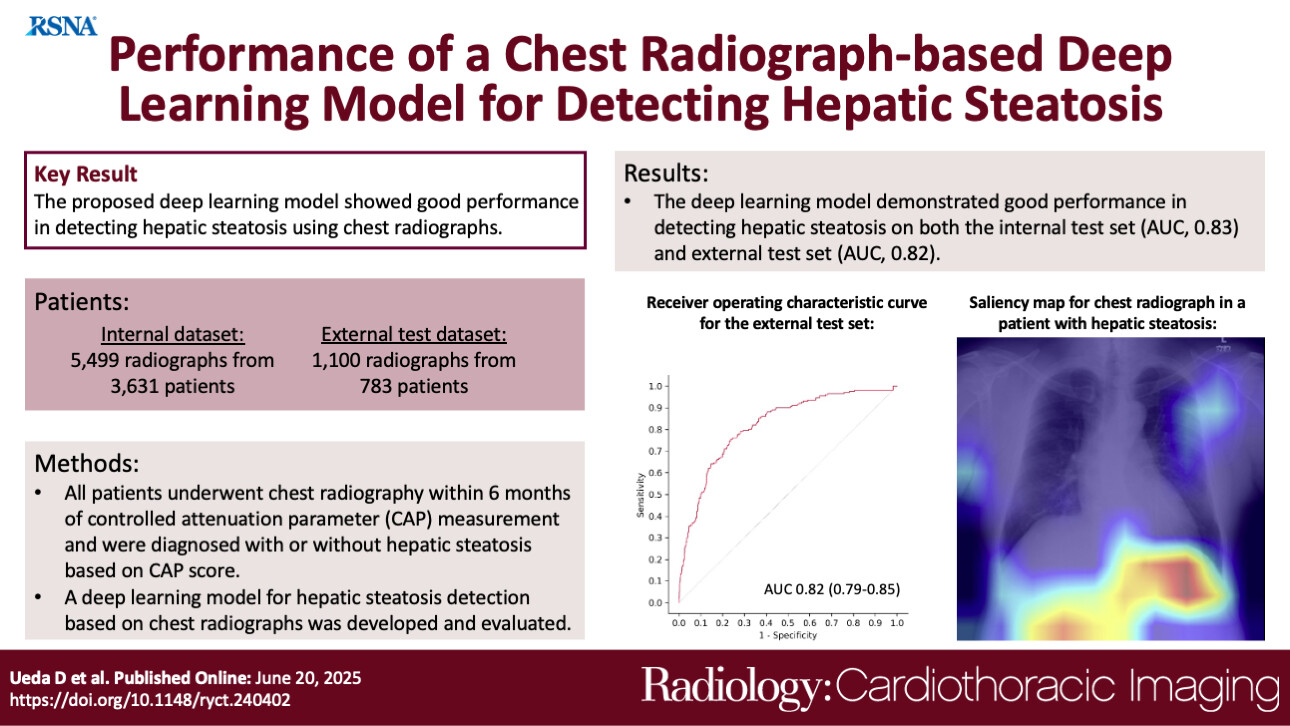
Hepatic steatosis, characterized by the accumulation of fat in the liver, is a condition that requires early detection as it can progress to cirrhosis and hepatocellular carcinoma. Currently, diagnostic methods such as ultrasound, CT, and MRI are used, but these face limitations regarding cost, accessibility, and operational effort. In this study, we focused on chest radiography, one of the most frequently performed imaging examinations in medical checkups and daily clinical practice. Since chest radiographs capture a portion of the liver, we developed a deep learning model to automatically detect hepatic steatosis from these images. We validated the model's accuracy using data collected from two different medical institutions.
Paper Details
This study utilized a total of 6,599 chest radiographs collected from two institutions. We used the "CAP value," an index obtained from transient elastography, as the reference standard (ground truth), defining hepatic steatosis as a value of 275 dB/m or higher. When evaluating the model's performance on a test dataset distinct from the training data (internal test set), the Area Under the Curve (AUC)—an indicator where a value closer to 1 represents higher performance—was 0.83. The sensitivity (the rate of correctly identifying those with the condition) was 68%, and the specificity (the rate of correctly identifying those without the condition) was 82%. Furthermore, in a validation using data from a completely independent external institution (external test set), the model maintained consistent performance with an AUC of 0.82, sensitivity of 76%, and specificity of 76%. Additionally, analysis visualizing where the AI focused within the images revealed that in approximately 74% of cases, the model prioritized the region around the liver and diaphragm as the basis for its judgment. The accuracy remained generally stable across patient subgroups, including differences in sex, obesity, and diabetes. While this was a retrospective study and further verification is needed for actual clinical application, the results demonstrate the potential of this method as a non-invasive screening tool.
