Research
Boron sensing and intracellular trafficking of boron transporters
Plant roots take up minerals and translocate them toward tissues where they are utilized. Among the 17 essential elements of plants, here we focus on boron (B) . B is required for structure of pectin in plant cell wall, but is toxic when present in excess. B deficiency and toxicity are both major agricultural problems worldwide. Therefore, understanding the molecular mechanisms underlying B transport is important to develop technology to alleviate B deficiency and toxicity problems. Using the model plant Arabidopsis thaliana, 2 types of B transporters facilitating boron transport across the plasma membrane (PM) have been identified (Fig.1). Under low B conditions, a boric acid channel, NIP5;1, and a borate exporter, BOR1, are required for efficient B uptake into roots and subsequent translocation toward shoots (Takano et al. 2002 Nature; Takano et al. 2006 Plant Cell; Fig. 1). Under high B conditions, expression of NIP5;1 and BOR1 is reduced, and a borate exporter BOR4 (Bot1 in barley) is responsible for B exclusion from the root (Miwa et al. 2007 Science).
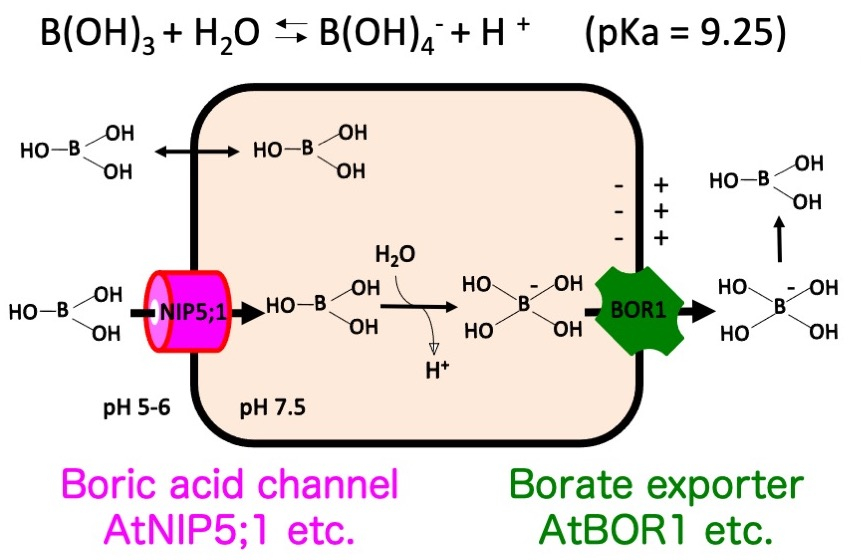
Fig. 1. Membrane transport mechanisms of boron (Yoshinari and Takano 2017 Frontiers, Mini Review)
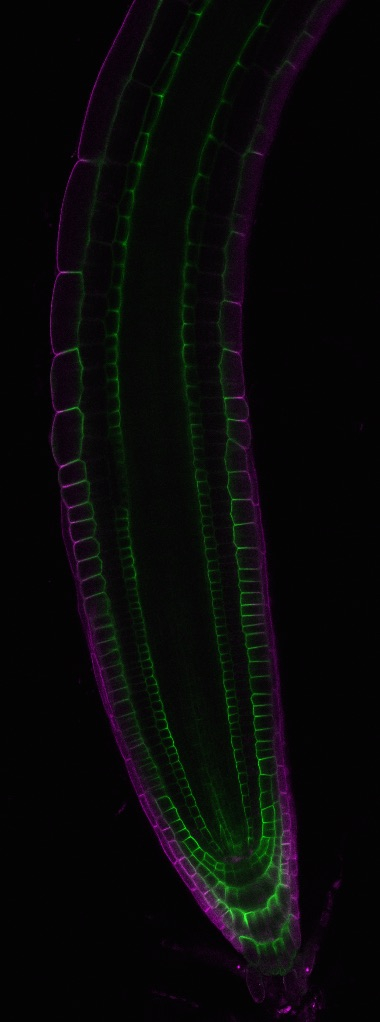
1. Polar localization of mineral transporters
Transporters should be localized in specific membrane domains in specific cells for efficient transport of minerals. Based on localization of fusion proteins with fluorescent proteins, NIP5;1 is preferentially localized in outer (facing the root surface) PM domains, while BOR1 is in inner (facing the center of roots) PM domains of various root cells (Takano et al. 2010 PNAS; Fig. 2). The polar localization of the boric acid channel and borate exporter supports radial transport of B from soil to inner portion of roots.
How is the polar localization maintained in plant cells? Our studies showed that the polar localization of NIP5;1 and BOR1 is not likely by static-retention mechanisms but is dependent on constitutive endocytosis (clathrin-mediated endocytosis) and recycling (Yoshinari et al. 2016 PCP; 2019 Plant Phys.;2021 Biol. Cell; Wang et al. 2017 Plant Cell; Fig. 3). We aim to understand the mechanisms behind the polar trafficking probably common to various mineral transporters.
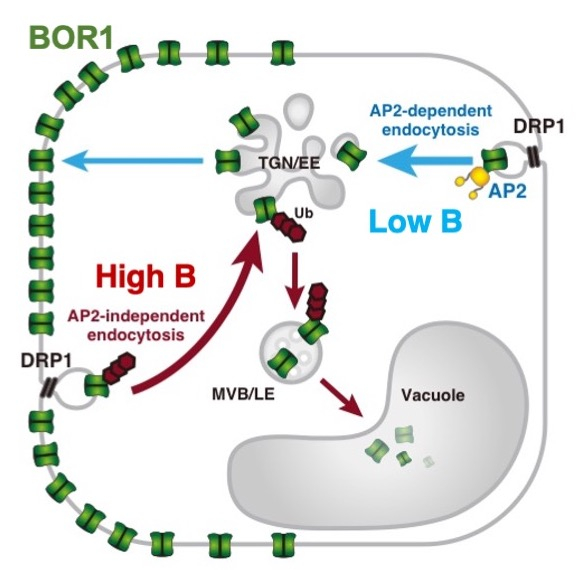
2. Boron sensing and endocytic degradation of a boron transporter (transceptor)
Activities of mineral transporters are often controlled by their substrates to maintain mineral homeostasis. As discussed above, BOR1 activity in the PM is required under low B conditions. However, upon high-B supply, it is quickly repressed through endocytic degradation of BOR1 (Takano et al. 2005 PNAS; Yoshinari et al. 2012 PSB). In responce to high B conditions, BOR1 is ubiqutinated, transferred from the PM to endosomes, and then degraded in the vacuole (Viotti et al. 2010 Plant Cell; Kasai et al. 2011 JBC; Fig. 3, 4). This rapid response is important to shut down B transport to avoid B toxicity (Aibara et al. 2018 Plant Phys).
How do plant cells sense B concentrations and regulate the fate of BOR1? Our study suggested that BOR1 is a B transceptor (transporter + receptor) directly senses the B concentration and promotes its own polyubiquitination and vacuolar sorting (Yoshinari et al. 2021 Plant Cell; Fig. 5). We aim to reveal the precise mechanisms of the transport-coupled ubiquitination of BOR1 and possibly other nutrient transceptors in plant cells.
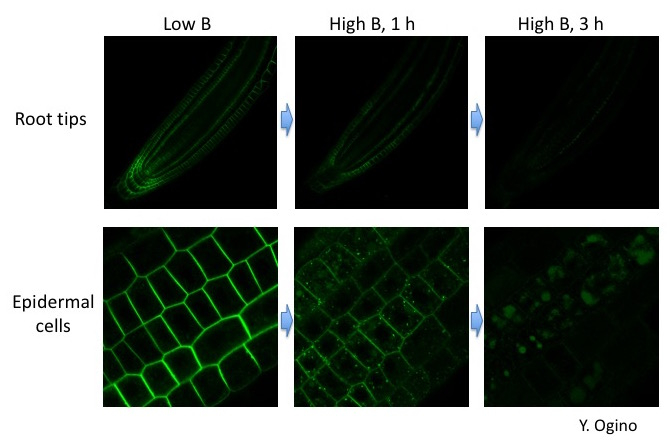
Fig. 4. High-B induced endocytosis and degradation of BOR1-GFP in root cells.
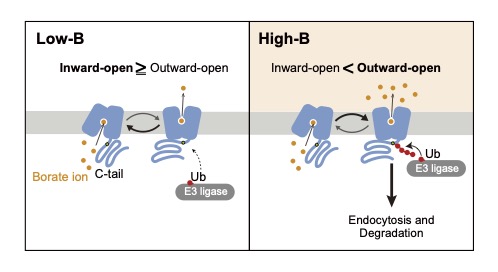
Fig. 5. A model for B sensing. A transport-ubiquitination coupled model. Under low-B conditions, BOR1 is largely in the inward-open state, with the K590 residue in the C-tail unexposed. Under high-B conditions, BOR1 is more frequently in the outward-open state, with the C-tail exposed, giving access to the K590 residue for E3 ligases. Longer exposure to the E3 ligase results in polyubiquitination and subsequent endocytic degradation of BOR1. (Yoshinari et al. 2021 Plant Cell)