Research News
Jan 22, 2026
- Engineering
Tuning color through molecular stacking: A new strategy for smarter pressure sensors
Initially stacked benzene layers increase fluorescent color change drastically when exposed to pressure, suggesting new ways to design the pressure sensors used in machinery and medical devices
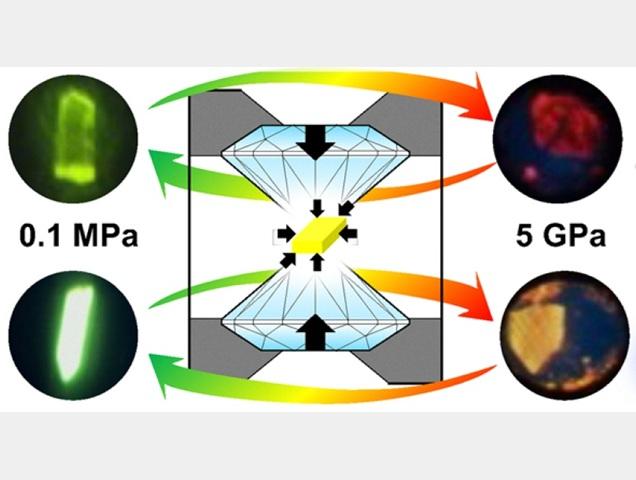
Pressure changes the color of two crystals from green to red by changing the closeness of the molecules
Squeezing the crystal changes how closely the molecules interact, which changes the color of light it emits from green at low pressure (left) to red at high pressure (right). In pCP-H (lower crystals), molecules naturally form close pairs and when pressure pushes these pairs even closer together, it strengthens the interactions between neighboring molecules and causes a large change in fluorescence color. In the other compound, pCP-iPr (upper), crystals do not form such pairs, resulting in a much smaller shift.
Credit: Osaka Metropolitan University
Piezofluorochromism, the phenomenon of materials reversibly changing their fluorescent color when pressure is applied, is used to create the pressure sensors used in automotive and medical industries. By monitoring color changes, researchers can visually recognize phenomena, such as chemical changes, that actually take place. However, as devices get increasingly complicated, there is an increasing demand for ways to produce more sensitive sensors.
A research group led by Project Assistant Professor Takuya Ogaki, Associate Professor Yasunori Matsui, and Professor Hiroshi Ikeda at the Graduate School of Engineering, Osaka Metropolitan University, has identified a new way to produce fluorescence, by finding that an initially stacked benzene layer (cyclophane moiety) increased its fluorescent color change drastically when exposed to pressure.
Professor Ogaki explained the background to the research. “It is difficult to rationally design organic crystals that exhibit the desired color change,” he said. “Even a slight change of the structure of organic molecules yields a completely different crystal structure.”
The researchers focused on two closely related crystalline organoboron compounds containing a special structural unit called [2.2]paracyclophane (pCP). When exposed to very high pressure, such materials show a shift in fluorescence toward longer wavelengths, resulting in them glowing red. Using X-ray crystallography, they found that the reason for this color change differed between the two crystals.
In one crystal, called pCP-H, the electron clouds naturally form pairs in stacked layers known as π-stacked dimer layers. Pressure pushes these pairs even closer together, strengthening the weak electron forces between neighboring molecules and causing a pronounced change in the fluorescent color.
In the other crystal, pCP-iPr, molecules do not form these stacked layers, so the color change mainly comes from subtle changes within the individual molecules making up the crystal, resulting in a much smaller shift and a less intense color.
“Under ultra-high-pressure conditions, we discovered that cyclophanes, such as [2.2]paracyclophane, act like springs, expanding and contracting to alter the luminescent color through changes in molecular interactions,” Professor Matsui explained.
Together, these results reveal that molecular pairing and the internal molecular structure affect how materials respond to pressure, providing valuable guidance for designing future pressure-sensitive materials.
Professor Ikeda concluded: “As materials function not only in molecular assemblies, like crystalline states, but also in monolayers, understanding both these processes is expected to become a new molecular design strategy.”
The findings were published in Journal of Materials Chemistry C.
Funding
This study was partially supported by: JSPS KAKENHI Grants (no. JP24H01092, JP22K14667, JP22H05377, JP20K15264, JP21H04564, JP22K05069, JP22K05147, JP16H06514, JP21K19029, JP17H06375, JP17H06371, JP18H01967, and JP21H05494); Konica Minolta Science and Technology Foundation, Tobe Maki Foundation (25-JA-016); Hattori Hokokai Foundation, Mayekawa Houonkai Foundation (A3-24006); 2024 Osaka Metropolitan University Strategic Research Promotion Project (Young Researcher); JST Grants of the establishment of university fellowships towards the creation of science technology innovation (no. JPMJFS2138); SPRING (no. JPMJSP2139).
Paper information
Journal: Journal of Materials Chemistry C
Title: The role of a [2.2]paracyclophane moiety in piezofluorochromism of crystalline organoboron complexes
DOI: 10.1039/D5TC03195H
Authors: Shun Irii, Takuya Ogaki, Shun Yamamoto, Hana Miyashita, Kazutaka Nobori, Hiroki Iida, Yoshiki Ozawa, Masaaki Abe, Hiroyasu Sato, Yasunori Matsui, Hiroshi Ikeda
Published: 20 October 2025
URL: https://doi.org/10.1039/D5TC03195H
Contact
Hiroshi Ikeda
Graduate School of Engineering
Email: hiroshi_ikeda[at]omu.ac.jp
*Please change [at] to @.
SDGs

