Research News
Jun 13, 2025
- Engineering
How solvents shape precision drug delivery
Solvent polarity controls drug-loading capacity of metal–organic framework carriers
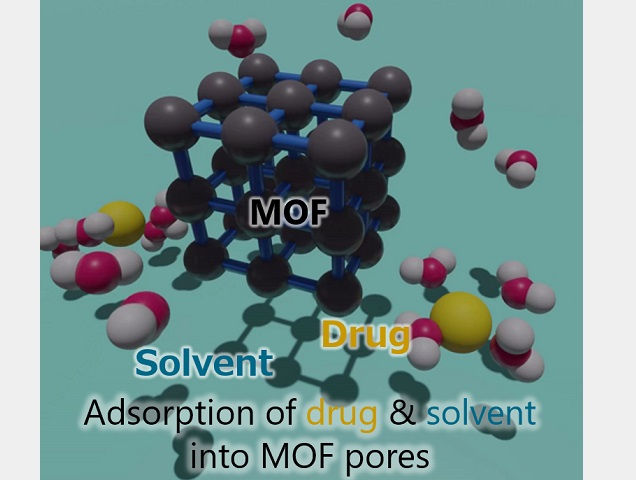
How drugs and solvents interact inside metal–organic framework (MOF) pores
Solvents play a critical role in boosting drug-loading capacity of MOFs.
Credit: Osaka Metropolitan University
Even the best products won’t meet expectations if they are packed poorly — packaging matters. The same goes for drug delivery.
Osaka Metropolitan University researchers have uncovered the critical role played by solvents in how effectively drugs can be loaded into metal–organic frameworks (MOFs), a promising class of drug carriers. Their findings shed light on a previously overlooked part of the loading process, with the potential to boost the efficiency of how medications are delivered inside the body.
To treat diseases effectively, it’s not just the medicine that counts — but also how that medicine is delivered into our bodies. Drug delivery systems (DDSs) are specially designed tools that help improve a drug’s effectiveness and safety by controlling the speed, timing and location of its release inside the body.
Composed of metal ions and organic ligands, MOFs are highly porous structures with extensive surface areas. Their unique architecture allows for high drug-loading capacity and targeted delivery, positioning them as next-generation DDS materials.
While the interaction between a drug and its MOF carrier is important, it is only part of the story.
“Most studies have focused only on how the drug interacts with the carrier, overlooking the important role that solvents play in the process,” said Shuji Ohsaki, an associate professor at Osaka Metropolitan University’s Graduate School of Engineering and lead author of this study.
To investigate this missing piece, the team studied how different solvents influence drug loading. They used two widely studied MOFs — ZIF-8 and UiO-66-NH₂ — as model carriers, and ibuprofen, a commonly used painkiller, as the model drug.
The team observed that ZIF-8 absorbed more ibuprofen in highly polar solvents, while UiO-66-NH₂ performed better in solvents with lower polarity.
Employing Raman spectroscopy to scrutinize these contrasting behaviors, the researchers found that solvent polarity influences molecular vibrations within the MOF structures. When these internal vibrations were suppressed, MOFs could absorb more of the drug.
“Our results show that solvent polarity doesn’t just influence how the drug dissolves — it actually changes how the MOFs behave at the molecular level,” Ohsaki said.
These findings highlight a physical link between solvent-induced changes in MOF dynamics and drug-loading performance. This link provides an important basis for selecting optimal solvents when designing MOF-based DDS platforms, which could enable more efficient and customizable treatments with fewer side effects.
“We plan to gain a more detailed understanding of the three interactions between MOFs, drugs and solvents through molecular simulation studies,” Ohsaki said.
The study was published in Langmuir.
Funding
- JSPS KAKENHI (Grant JP23KJ1849),
- Hosokawa Powder Technology Foundation (Grant HPTF22504),
- 2024 Osaka Metropolitan University (OMU) Strategic Research Promotion Project (STEP-UP Research).
Paper information
Journal: Langmuir
Title: Influence of Solvents on Drug Loading Capacity of Metal–Organic Frameworks Focusing on Solvent Dipole Moment
DOI: 10.1021/acs.langmuir.4c04896
Authors: Kazuki Ohshima, Keisuke Mizomichi, Shuji Ohsaki*, Hideya Nakamura, Satoru Watano
Published: March 18, 2025
URL: https://doi.org/10.1021/acs.langmuir.4c04896
Contact
Shuji Ohsaki
Graduate School of Engineering
Email: shuji.ohsaki[at]omu.ac.jp
*Please change [at] to @.
SDGs
