Research News
Nov 11, 2025
- Medicine
Anti-Amyloid Therapy Does Not Change Short-Term Waste Clearance in Alzheimer’s
After lecanemab treatment, MRI scans show no short-term change in waste clearance function in Alzheimer’s patients
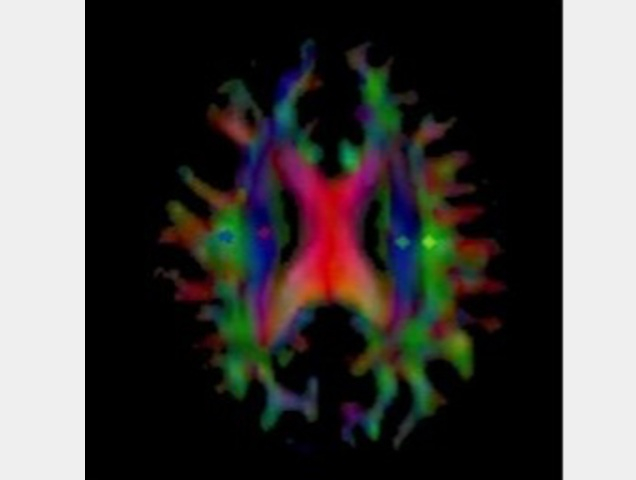
Map of an Alzheimer's patient's brain showing the diffusion of water as part of glymphatic flow
MRI scans were performed before and after the initiation of treatment for Alzheimer's disease patients, and the DTI-ALPS index was calculated. Colors show regions of interest in the brain.
Credit: Osaka Metropolitan University
A group from Osaka Metropolitan University in Japan, led by graduate student Tatsushi Oura and Dr. Hiroyuki Tatekawa, found that treatment using the drug lecanemab to remove amyloid plaques in the brain does not change the waste clearance function in the brains of Alzheimer’s disease (AD) patients in the short term.
This suggests that even after treatment, the AD patients’ nerves are already damaged, and the waste clearance function does not recover in the short term. Their findings show the complexity of the disease and the need to address multiple disease-causing pathways simultaneously in the future.
Their findings add to the complicated process of unraveling AD’s mechanisms. Despite being the most common form of neurodegenerative disease, it is tricky to treat because of its multiple causes.
One cause of the nervous damage common in AD is the accumulation of the protein amyloid-β (Aβ) in the brain. In healthy patients, the glymphatic system moves cerebrospinal fluid along the spaces around the arteries into the brain tissue, where it mixes with interstitial fluid to carry away metabolic waste like Aβ. This is called the ‘glymphatic system’ after the glial cells involved in the process.
However, in AD patients, Aβ builds up, stiffening arteries and reducing the flow from the brain to the cerebrospinal fluid. This blockage triggers a chain of neurodegenerative processes, leading to AD symptoms.
The recently approved therapeutic lecanemab reduces accumulated Aβ. The team from the university’s Graduate School of Medicine evaluated the glymphatic system before and after treatment in patients who received lecanemab therapy, using the DTI-ALPS index.
Contrary to expectations, they found no significant change in the index between pre-treatment and 3 months after treatment.
They concluded that although anti-amyloid therapy can reduce plaque burden and slow further cognitive worsening, it may be insufficient to restore lost function. This suggests that neuronal damage and clearance system deficits have already been well established by the time the patient starts showing symptoms. Their findings show the range of factors involved in the progression of AD, many of which are not easily reversible.
“Even when Aβ is reduced by lecanemab, impairment of the glymphatic system may not recover within the short-term,” Oura said. “In the future, we want to look at factors like age, the stage of the disease, and degree of lesions in the white matter to further understand the relationship between changes in the glymphatic system due to lecanemab treatment and the outcome of treatment. This will help understand the best way to administer treatment to patients.”
The study was published in Journal of Magnetic Resonance Imaging.
Funding
This study was supported by the Takeda Science Foundation (ROR ID: 02y123g31) and Japan Society for the Promotion of Science (JSPS) KAKENHI (grant number: 25K19115).
Paper information
Journal: Journal of Magnetic Resonance Imaging
Title: Unchanged Early Diffusion Tensor Imaging Along Perivascular Space Index After Amyloid-Targeting Disease-Modifying Therapy in Alzheimer's Disease: A Preliminary Study
DOI: 10.1002/jmri.70118
Authors: Tatsushi Oura, Daisuke Horiuchi, Hiroyuki Tatekawa, Hirotaka Takita, Akitoshi Takeda, Taro Shimono, Ayako Omori, Daiju Ueda, Natsuko Atsukawa, Yoshiaki Itoh, Shu Matsushita, Yukio Miki
Published: 8 September 2025
URL: https://doi.org/10.1002/jmri.70118
Contact
Tatsushi Oura
Graduate School of Medicine
Email: sx23812o[at]st.omu.ac.jp
*Please change [at] to @.
SDGs


